
Gel-Pac® Permits Combining AviPro® Megan® Egg Salmonella Vaccine and
Coccidiosis Vaccine for Hatchery Spray Application
SUMMARY
A principal method of reducing Salmonellosis in poultry and the vertical transmission of Salmonella to poultry products for humans includes administering a live Salmonella vaccine at one day of age in the hatchery. Operators have not been able to successfully combine live Salmonella vaccines with coccidiosis vaccine due to the inactivation of Salmonella vaccine by antagonistic additives in coccidiosis vaccines. This report details the negative impact of this antagonism and demonstrates a process to overcome it, allowing hatcheries to combine Salmonella vaccine with a coccidiosis vaccine in one application of stabilized edible gel beadlets. Gel delivery is proven to be a more effective vehicle for gut-protective vaccines; this process allows the hatchery to vaccinate chicks or poults against both diseases simultaneously, and ensure the birds receive a fully protective dose targeted directly at the gut, where both vaccines initiate immune stimulation.
DESCRIPTION OF PROBLEM
Human populations are growing in number and in affluence; creating a literal hunger for more food, and healthier diets. Improving food safety is one goal that spurs workers to improve animal production practices. The effort is global in scope, sprouting from governments’ desires to protect citizens and protect the reputation of their meat exports. Shoulder-to-shoulder with governments, multinational animal health and integrated food companies also work to be valued for their role in protecting animal health and, consequently, food safety. Protection from Salmonella tops the agenda, and the list of tactics to reduce the Salmonella burden from poultry products includes maintaining biosecurity, monitoring pathogens, selectively using antimicrobial drugs, and defending gut health through probiotics, prebiotics and vaccinations.1 This research report focuses on making vaccination more convenient as well as more effective.
The weight of more consumers preferring meat from antibiotic-free animals, plus government regulations to reduce animal antibiotic treatments, have tipped a balance; it puts more pressure on Salmonella prevention rather than cure. Prevention is the role uniquely suited to vaccines, helping animals to build their own defenses rather than using medications to correct sickness after the fact.
Live Salmonella vaccines work. AviPro® Megan® Egg and AviPro® Megan® Vac,1 attenuated live Salmonella vaccines, reduce the Salmonella load in chickens and turkeys – a benefit that is measured in significantly reduced Salmonella in live birds,2-4 and significantly less contamination in processing plant pre-evisceration, post-chill, and parts rinse when birds are vaccinated at day 1 in the hatchery and again at day 14.5 Importantly, the bird’s gut on day 1 is at its cleanest. Like a freshly cultivated field, the new gut is virtually free from contamination. It is fertile ground, allowing the Salmonella vaccine to seed itself and occupy the gut to defend the young bird. The vaccine locally immunizes the chick or poult on the mucosal surface, and initiates cell-mediated systemic immunity for internal organs. These defenses protect the gut tissue and the internal organs from pathogens that are sure to come after the chick or poult arrives at the farm.6, 7
The AviPro Megan vaccines’ mode of action is to first colonize the gut, then to stimulate cellular, local and systemic immunity. An accepted means of vaccinating with the Salmonella vaccines at one day of age in hatcheries is via coarse spray, using cabinets with aerosol spray nozzles similar to those used to vaccinate against bronchitis, Newcastle disease, and coccidiosis.
Coccidiosis vaccines, like Salmonella vaccines, are among those that can be sprayed on chicks and poults or consumed by the birds in drinking water or feed. Researchers reported that coccidiosis vaccines administered in sprayed edible gel droplets protected significantly better than the same vaccine in liquid spray.8, 9 In addition to edible gel being an intuitive way to deliver an oral vaccine or other oral additive, gel has been shown to uniformly suspend vaccine without constant agitation, induce less evaporative heat loss from chicks,10 improve vaccine uniformity, and reduce vaccine waste.11
Edible gel droplet applicators, either newly installed or retrofitted from existing cabinet sprayers, are placed in-line at the hatchery. Early tests with gel droplets used gels prepared by sequential heating, forming and cooling.8 Newer versions of gel require little preparation. A cool-water soluble gel powder is homogenized with approximately 97% hatchery tap water, then the vaccine is uniformly mixed in. Hatchlings, sprayed while being moved through the hatchery, eat the gel droplets within a few minutes, giving the oral additive direct access to the gut before the bird leaves the hatchery. Protection begins before the bird arrives at the farm.
It sounds simple, spray an edible gel vaccine in the hatchery to gain the upper hand on Salmonella. Even more convenient if the two most commonly-used vaccines to address Salmonella and coccidiosis could be mixed and consumed in the same gel. Such mixing is possible, but one must take deliberate care to prevent the antagonistic preservatives in most coccidiosis vaccines from killing the Salmonella vaccine and ruining this critical first step in Salmonella control. It is a dominant practice among coccidiosis vaccines to include powerful preservatives; these preservatives are lethal to other biologicals, including Salmonella and IB/ND vaccines, unless the vaccines are properly stabilized. One edible gel delivery system, Gel-Pac®, is engineered to protect fragile biologicals from antagonistic coccidiosis vaccine. Gel-Pac has been observed to reduce oxidation attributed to 4 commercial coccidiosis vaccines preserved with potassium dichromate or chloramine,12 and is reported to stabilize IB vaccine and probiotic bacteria against inactivation by chlorine, iron and nitrate.13
MATERIALS AND METHODS
To assess the protective effect of Gel-Pac toward AviPro® Megan® Egg, an attenuated live Salmonella vaccine, researchers14 prepared 3 vaccine solutions and measured the Salmonella vaccine titer retention in each solution throughout 6 hours. The vaccine solutions were:
- Salmonella Vaccine (108 CFU/ml) in water, as control
- Salmonella vaccine (108 CFU/ml) plus coccidiosis vaccine (100 doses/ml) in water, as a non-stabilized solution
- Salmonella vaccine (108 CFU/ml) plus coccidiosis vaccine (100 doses/ml) in water stabilized with Gel-Pac (25g/l), as a gel-stabilized solution
Throughout the 6-hour period, the Salmonella vaccine titers were determined in triplicate at 0, 0.5, 1, 2, 4, and 6 hours following mixing.
aAviPro® Megan® Egg, Elanco Animal Health, Greenfield, IN 46140 USA
bHATCHPAK® COCCI III, Boehringer Ingelheim, Gainesville, GA 30503, USA
cGel-Pac,® Animal Science Products, Inc., Nacogdoches, TX 75963, USA
RESULTS
There were no significant differences in vaccine concentrations among the treatments at the initial and 30 minute applications. After 1 hour in solution, the gel-stabilized vaccine maintained greater concentrations than both the control (p<0.01) and non-stabilized vaccine (p<0.05), the gel-stabilized vaccine being 108.42 CFU/ml, control vaccine 108.11 CFU/ml, and non-stabilized vaccine 108.19 CFU/ml (Table).
The trend in the decay of non-stabilized vaccine continued, the titer falling to 107.72 CFU/ml at 4 hours, remaining significantly less than control at 108.11 CFU/ml (p<0.05) and stabilized gel at 108.29 CFU/ml (p<0.01). The respective concentrations, ranked and expressed as percentages of the initial dose for gel-stabilized, control, and non-stabilized vaccines were 88%, 80%, and 34% (Figure).
The general pattern of Salmonella vaccine preservation in gel and inactivation in the non-stabilized solution continued through the 6-hour sampling. Stabilized gel maintained 108.23 CFU/ml, significantly more than non-stabilized vaccine at 107.86 CFU/ml (p<0.01). Control vaccine titer was intermediate at 108.08 CFU/ml. The respective concentrations for gel-stabilized, control, and non-stabilized vaccine were 80%, 76%, and 47%, expressed as percentages of the initial dose. Throughout the entire study, the Salmonella vaccine in the non-stabilized application exhibited a markedly accelerated decay leading to significantly reduced final titer, compared to the gel-stabilized solution (Figure). The rate of titer decay among gel-stabilized and control applications was similar at -3.7% per hour and -4.5% per hour, respectively; the Salmonella vaccine decay rate in non-stabilized spray more than doubled to -10.5% per hour.
CONCLUSIONS AND APPLICATIONS
The significant deterioration experienced by the Salmonella vaccine in the presence of coccidiosis vaccine was completely prevented by Gel-Pac’s stabilizing properties. Safely combining the two vaccines in one application offers hatcheries the potential to efficiently vaccinate with one spray. Furthermore, the edible gel droplets facilitate chicks or poults receiving the vaccine directly targeted to the gut, where both vaccines must inhabit before beginning their protective work. Edible gels carrying coccidiosis vaccine have been shown to protect the gut significantly better against coccidiosis challenges than liquid spray.8, 9 Live Salmonella vaccine’s mode of action is also to first colonize the gut, before traversing into the reticuloendothelial system, where cellular, local and system immunity are stimulated. This research confirms Gel-Pac rescues live Salmonella vaccine from the antagonistic effect of coccidiosis vaccine, offering hatcheries an efficient, convenient way to administer both vaccines.
It is a great time to be in agriculture. Our growing population requires more food, while demanding it to be healthy and safe. Those demands require heavy lifting on our part; in the case of preventing Salmonellosis using live vaccines, the hatchery is uniquely positioned to take the critical first step.
Table. Salmonella vaccine concentrations in water alone, non-stabilized coccidiosis vaccine solution, and coccidiosis vaccine solution stabilized with Gel-Pac.®
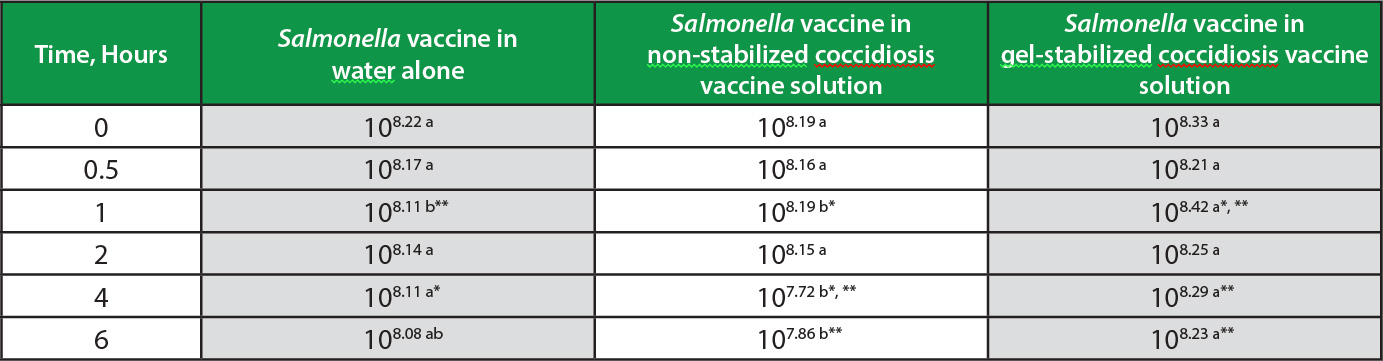
Concentration (CFU/ml) of Salmonella vaccine (initial dose 108 CFU/ml) after being held in solutions of water as control, water with coccidiosis vaccine (100 doses/ml), and water with coccidiosis vaccine (100 doses/ml) stabilized with Gel-Pac (25g/l). Mean of 3 replicates per time point.
ab Within sampling time, means sharing lowercase letters are not significantly different.
*, ** Different lowercase letters indicate significance using * (p<0.05) and ** (p<0.01).
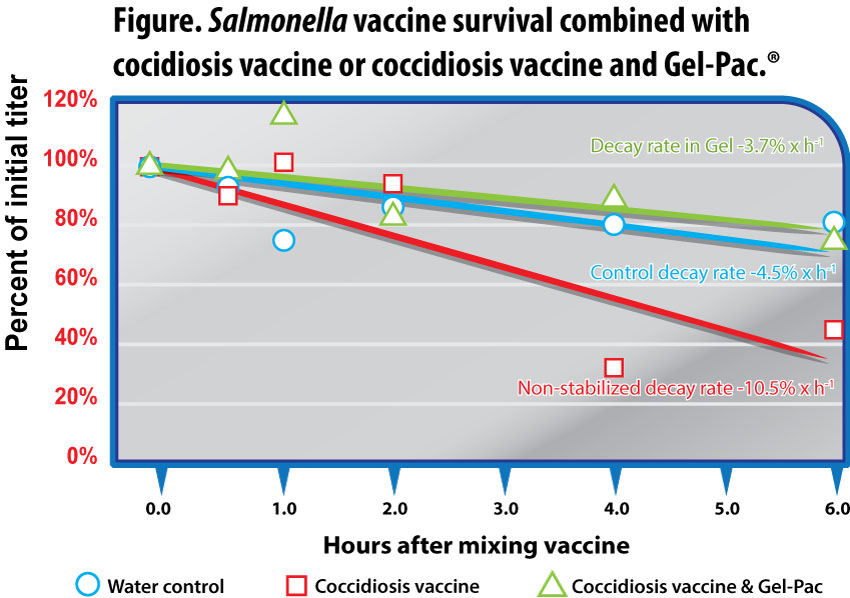
Concentration of Salmonella vaccine as a percent of initial titer (108 CFU/ml added) after being held in solutions of water as control, water with coccidiosis vaccine (100 doses/ml), and water with coccidiosis vaccine (100 doses/ml) stabilized with Gel-Pac (25g/l). Mean of 3 replicates per time point.
REFERENCES AND NOTES
1. Van Oort, R. Salmonella control: A global perspective. PoultryWorld. Accessed Apr 2018. http://www.poultryworld.net/Special-Focus/Salmonella-special/Salmonella-control-A-global-perspective/
2. Aehle, S. 2017. Salmonella control in broilers. Elanco Animal Health USPBUMV100011. Accessed May 2018. https://www.elanco.us/products-services/poultry/salmonella-control
3. McComb, B. 2017. Salmonella control in turkeys. Elanco Animal Health USPBUMEG00007. Accessed May 2018. https://www.elanco.us/products-services/poultry/salmonella-control
4. Aehle, S. 2017. Control of Salmonella in live broiler production. USPBUMV100001(1). On file, Elanco Animal Health.
5. Aehle, S. 2018. Two plants/three grow-out cycles: Broiler field trial results. Paper presented at the Tech Talk, International Production & Processing Expo (IPPE), Atlanta, Georgia.
6. Trampel, D.W., T.G. Holder and R.K. Gast 2014. Integrated farm management to prevent Salmonella enteritidis contamination of eggs. JAPR. 23:353-365.
7. Hassan, J.O. and R. Curtiss III. 1994. Development and evaluation of an experimental vaccination program using a live avirulent Salmonella typhimurium strain to protect immunized chickens against challenge with homologous and heterologous Salmonella serotypes. Infect. Immun. 62:5519-5527.
8. Jenkins, M.C. et al. 2012. Gel-bead delivery of Eimeria oocysts protects chickens against coccidiosis. Avian Dis. 56:306–309.
9. Firouzi, N. et al. 2014. Efficacy of anticoccidial vaccination of chickens via different routes: a comparative study. Bulgarian Journal of Veterinary Medicine, On-line first. ISSN 1311-1477, http://tru.uni-sz.bg/bjvm/bjvm.htm
10. Cargill, P. 2014. Personal communication. Gel-Pac hatchery spray vaccination. Pharmsure International, Ltd. Accessed May 2018. https://pharmsure.net/app/uploads/2016/12/Pharmsure-Gel-Pac-hatchery-spray-vaccination.pdf
11. Alonzo, C. 2014. Using gel vaccination in the hatchery for coccidiosis control. WATTAgNet.com. Accessed May 2018. https://www.wattagnet.com/articles/19319-using-gel-vaccination-in-the-hatchery-for-coccidiosis-control.
12. Izard, R. 2016. Gel-Pac Enables hatchery vaccination with IB and coccidiosis in combination. Animal Science Products, Inc. Accessed May 2018. https://www.asp-inc.com/wp-content/uploads/2016/08/Gel-Pac%C2%AE-Enables-Hatchery-Vaccination-with-IB-and-Coccidiosis-in-Combination.pdf
13. Izard, R. 2015. Unpublished data.
14. Aehle, S. 2017. Personal communication. Compatibility test(s) performed by third party laboratory and results provided by Elanco Animal Health.
Additional Information and Related Articles on Gel-Pac®
SpecSheet | Spec Sheet Spanish | Sell Sheet | SDS | SDS Chinese | SDS Spanish Gel-Pac® new generation stabilizer aids in the administration of gel-delivered vaccines and probiotics for poultry. It is especially designed to prolong the viability and improve the effectiveness of reconstituted and diluted vaccines and probiotics in a gel that is convenient to prepare and easy for birds to consume.
RELATED ARTICLES:
Gel-Pac® Permits Combining AviPro® Megan® Egg Salmonella Vaccine and Coccidiosis Vaccine for Hatchery Spray Application PDF | ARTICLE
Gel delivered vaccines, probiotics, and nutritional ingredients for poultry PDF | ARTICLE
Gel-Pac Technical Report PDF | ARTICLE
Gel-Pac Streamlines Newcastle Vaccine and Oral Additive Delivery PDF | ARTICLE
Gel-Pac Improves the Titer of Live Infectious Bronchitis PDF | Spanish PDF
Gel-Pac Improving the titer of live infectious bronchitis vaccine for hatcheries PDF / ARTICLE
Gel-Pac Efficacy of Gel-Pac Application for Poultry Coccidiosis Vaccination PDF | ARTICLE
Gel-Pac Enables Hatchery Vaccination with IB and Coccidiosis in Combination PDF | Chinese PDF | ARTICLE
Gel-Pac Delivering Optimal Suspension PDF | Spanish PDF| ARTICLE
Mailing Address:
Animal Science Products, Inc
PO Drawer 631408
Nacogdoches, TX 75963 - 1408
Physical Address:
3418 Rayburn Drive
Nacogdoches, Texas 75961
Phone
936.560.0003
