 EFFICACY EVALUATION OF MUCUSOL SOLUTION IN DRINKING WATER OF PIGS EXPOSED TO H1N1 SWINE INFLUENZA VIRUS AND PASTEURELLA MULTOCIDA
EFFICACY EVALUATION OF MUCUSOL SOLUTION IN DRINKING WATER OF PIGS EXPOSED TO H1N1 SWINE INFLUENZA VIRUS AND PASTEURELLA MULTOCIDA
Who doesn’t like a free ride?
Picture moving sidewalks…you see them in airports throughout the world…there are two ways to use them. You notice some people who like to stand and enjoy the free ride, being carried leisurely along. Others stroll or even run on the moving sidewalk, accelerating their progress while being whisked productively on their way. If you are the second type, a walker, you may be frustrated by the other slow-movers plugging the path. Now think of the respiratory tract; like a moving sidewalk, cilia normally whisk mucus easily along. You hardly notice mucus unless, like other slow movers, it plugs your path. When infection or irritation hit, mucus becomes thick and elastic, choking the airway.
People have historically used expectorants to help make mucus thinner, less sticky, and easier to expel. Colonial explorers found natives in the tropical Americas using Guaiac tree extracts in the 16th century. Certain species of these trees still serve as the national tree of The Bahamas and the national flower of Jamaica. More recently gum of the Guaiac, which is approved in Europe and the U.S. as a food additive, was refined and eventually synthesized as guaifenesin, present as an active ingredient in the expectorant MucuSol®.
Human medical researchers worked to demonstrate how guaifenesin functions in the petri dish (Seagrave et al., 2012). When dosed on human respiratory cells, guaifenesin caused the cells’ mucus to become thinner and less elastic. Their videos even visually show cilia moving mucus more quickly. This work supported what those early explorers experienced…guaiac cleared mucus and the trapped irritants more easily. Coughs became more productive and respiratory symptoms less severe.
Extending The Model To Animals
More recently, Lechtenberg et al. (2016) have taken the technology out of the petri dish and into the pig, using their Swine Influenza Virus (SIV) challenge model to measure some of MucuSol’s effects during a severe respiratory challenge. The work was carried out at Central States Research Centre, Inc., Oakland, NE.
Materials and Methods
Twenty-five healthy, 3- 4-week-old crossbred pigs of either sex were confirmed SIV negative before arriving to the study site. The pigs were without therapeutic antimicrobial by any route 14 days prior to challenge and had no history of SIV or P. multocida vaccination. Pigs acclimated for 12 days before being challenged. Study animals were randomized to placebo and treatment groups and housed in one BSL-2 (5600 series) room. Pigs were provided water ad libitum throughout the study period, and were fed a grower ration that did not contain therapeutic antimicrobials.
Twenty study pigs were challenged on day 0 intra-nasally with 4.0 mL containing 1×105.5TCID50/mL of H1N1 SIV. On day 3, study pigs were secondarily challenged intra-nasally with 4.0 mL containing 1×108 CFU/mL of P. multocida.
The clinical signs of infection using this model appear 1-3 days after SIV challenge. To mimic the field application of MucuSol expectorant, presented as a guaifenesin-containing syrup, the treatment group received 7.4 mL per gallon of drinking water beginning on day 2, at the appearance of symptoms. The placebo group received water alone, and both treatments continued until all pigs were euthanized on day 8. A general health observation was conducted twice daily on all study animals. Any abnormality prompted the animal caretaker to notify the staff veterinarian. Intervention was provided by a veterinarian in consideration of the objectives of the protocol and in compliance with SOPs on animal care and use. Clinical scoring and rectal temperature collection started daily pre-challenge on day 0 and continued daily until euthanasia on day 8. Average respiratory score, nasal secretion score, depression score, and rectal temperatures were calculated for each treatment group for days 0-8.
Pigs were scored on the following scale:
Nasal Secretion Assessment
0 = Normal – moist nares, no abnormally excessive discharge
1 = Mildly abnormal – increased serous nasal discharge (clear)
2 = Moderately abnormal – increased viscous nasal discharge (cloudy)
3 = Severely abnormal – purulent nasal discharge (yellow +/- blood tinged)
Respiratory Score
0 = Rate and pattern normal, no abnormal nasal discharge
1 = Mild – slightly increased respiratory rate, some roughness in breathing
2 = Moderate – increased respiratory rate, some abdominal breathing
3 = Severe – increased respiratory rate with abnormal effort, open mouth breathing, grunting, dog sitting
Depression Score
0 = Normal – alert, active, normal appetite, well-hydrated, coat normal
1 = Mild – moves slower than normal, slightly rough coat, may appear lethargic but upon stimulation appears normal
2 = Moderate – inactive, may be recumbent but is able to stand, gaunt, may be dehydrated
3 = Severe – down or reluctant to get up, gauntness evident, dehydrated
Any animal that died during the study was subjected to a necropsy and required samples were collected. Upon day 8 necropsy, individual lung lobes were evaluated and the amount of consolidation was recorded. Lung lesions were differentiated by apparent etiology.
Figure 1
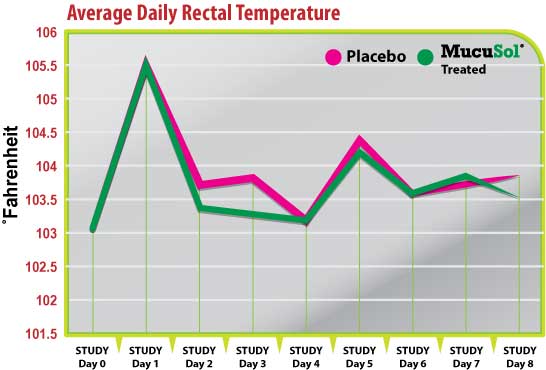
Figure 2
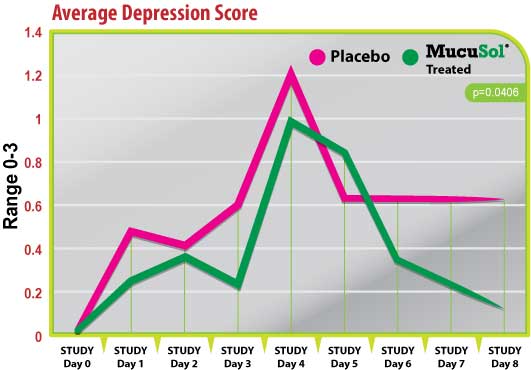
Figure 3
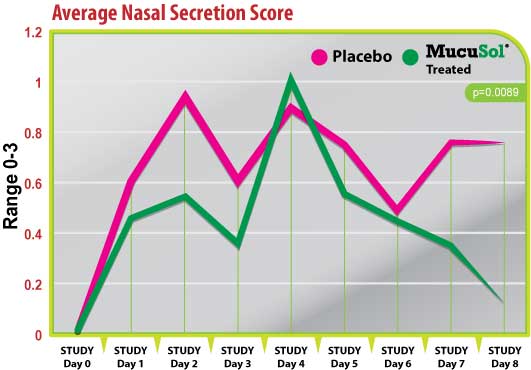
Figure 4
Results & Discussion
Rectal temperatures followed the same trends for each treatment group during most of the study (Fig. 1). Each group had a peak in average rectal temperature on day 1 (1-day post-SIV challenge), and a smaller peak in average temperature on day 5 (2-days post-P. multocida challenge). The average temperatures in the placebo group did not reduce as quickly after the SIV challenge as the MucuSol treated group did. On day 2 the average rectal temperature for the placebo group was 103.7˚F while it was 103.3˚F in the MucuSol treated group (p=0.0467). On day 3 the average rectal temperature for the placebo group was 103.8˚F, while the average temperature for the MucuSol treated group was 103.3˚F (p=0.0562).
Both treatment groups started with an average nasal secretion score of 0 (Fig. 2). Nasal secretion scores were generally lower in the MucuSol treated group than in the placebo group. Both treatment groups had a significant increase in nasal secretion score average on days 1 and 2 (post-SIV challenge), and day 4 (1-day post-P. multocida challenge). The placebo group had a greater average nasal secretion score on day 1 than the MucuSol treated group (placebo: 0.60 vs. MucuSol: 0.44), and on day 2 (placebo: 0.90 vs MucuSol: 0.56). The MucuSol treated group had a higher average nasal secretion score on day 4 than the placebo group (placebo: 0.89 vs MucuSol: 1.00).
The MucuSol treated group reduced in average nasal secretion score more quickly than the placebo group did after each challenge (day 3 placebo: 0.60, MucuSol: 0.33; day 5 placebo: 0.75, MucuSol: 0.56).
The MucuSol treated group also showed signs of overall resolution of nasal secretions towards the end of the study, while the placebo group did not. Lower secretion scores in the MucuSol treated group tended toward statistical significance on day 7 (placebo: 0.75 vs MucuSol: 0.33, p=0.0962). A statistically significant difference was found in the average scores on day 8 with the MucuSol treated group having the lower nasal secretion scores (placebo: 0.75 vs MucuSol: 0.13, p=0.0089).
Both treatment groups started with average depression scores of 0 on day 0 (Fig. 3). Both groups had elevations in average depression scores on day 1 (post-SIV challenge) and day 4 (1-day post-P. multocida challenge). The placebo group had a greater elevation of depression scores after the SIV challenge and the P.multocida challenge than the MucuSol treated group (day 1 placebo: 0.50 vs MucuSol: 0.22; day 4 placebo: 1.22 vs MucuSol: 1.00).
The MucuSol treated group tended to show a reduction in depression scores prior to the P. multocida challenge (day 3) while the placebo group did not (placebo: 0.60 vs MucuSol: 0.22, p=0.1065). The MucuSol treated group also showed a continual reduction in average depression score towards the end of the study, which reached significance on day 8, while the placebo group did not (day 7 placebo: 0.63 vs MucuSol: 0.22, p=0.1035; day 8 placebo: 0.63 vs MucuSol: 0.13, p=.0406).
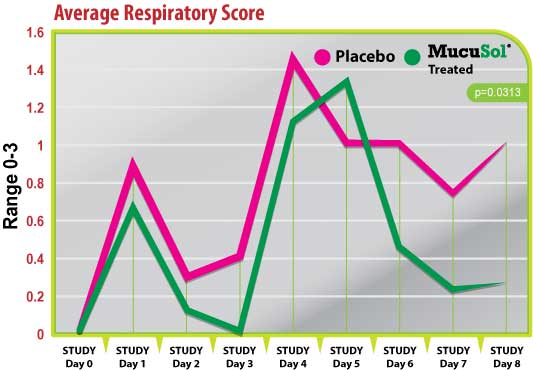
Both treatment groups started with average respiratory scores of 0 on day 0 (Fig. 4). Both groups responded with a peak in respiratory scores after the SIV challenge (day 1), and after the P. multocida challenge (day 4) with the placebo group having a higher average score than the MucuSol treated group on both days (day 1 placebo: 0.90 vs MucuSol: 0.67; day 4 placebo: 1.44 vs MucuSol: 1.11).
There was a statistically significant difference between the treatment group average respiratory scores on day 3 (placebo: 0.40 vs MucuSol: 0.00, p=0.0332). The MucuSol treated group did have a higher average respiratory score than the placebo group on day 5 (placebo: 1.0 vs MucuSol: 1.33). However, the MucuSol treated group displayed a continual reduction in respiratory scores after that day, while the placebo group did not (day 6, 7, 8 placebo: 1.00, 0.75, 1.00, respectively vs MucuSol: 0.44, 0.22, 0.25, respectively). Scores were tending toward statistical difference on day 7 (p=0.0810), and became significant on day 8 (p=0.0313).
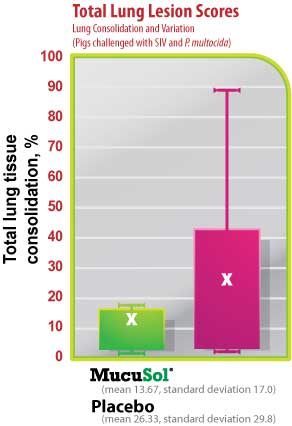
The average SIV lung lesion score (Fig. 5) was higher in the placebo group than in the MucuSol treated group (placebo: 8.0 vs MucuSol: 5.7). The average P. multocida lesion score (Fig. 6) was also higher in the placebo group than in the MucuSol treated group (placebo: 18.3 vs MucuSol: 8.0). The total lung lesion score (Fig. 7) was consequently higher in the placebo group than in the MucuSol treated group (placebo: 26.30 vs MucuSol: 13.70).
Lung scores exhibited more uniformity in the MucuSol treated group for total lung score, SIV lung lesion average, and P. multocida lesion average. No statistical difference was found between the lung lesion scores.
Conclusion
This study demonstrated interesting trends and some significant effects of MucuSol at diminishing specific clinical parameters associated with a SRD complex of viral and bacterial pathogens, and may serve as the basis for more statistically robust studies. Previous experiments in human cell cultures established that guaifenesin reduced mucus production, viscosity, and elasticity in a dose-dependent manner. The water-miscible MucuSol syrup used in this study facilitated the delivery of the normally-insoluble active ingredient by drinking water. MucuSol’s label recommendation and the dose chosen for the present study were established to proportionately approximate the OTC human dosage, and the results suggest it is in the range to obtain measurable respiratory responses in pigs.
Figure 5
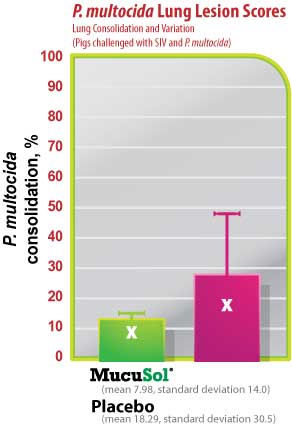
Figure 6
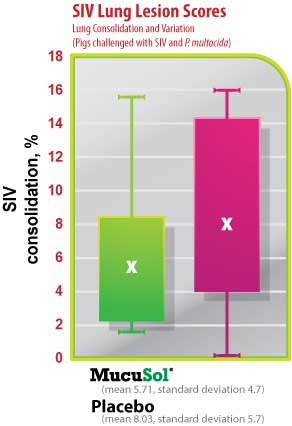
Figure 7
References
Lechtenberg, K., et al., 2016 (Unpublished). Proof of Concept Efficacy Evaluation of MucuSol® solution in drinking water of pigs exposed to H1N1Swine Influenza Virus and Pasteurella multocida.
Seagrave, J., Albrecht, H.H., Hill, D.B., Rogers, D.F. and Solomon, G., 2012. Effects of guaifenesin, N-acetylcysteine, and ambroxol on MUC5AC and mucociliary transport in primary differentiated human trachealbronchial cells. Respiratory Research, 13:98.
Additional Information and Related Articles on Uni-Sol®
SpecSheet English | SDS | SpecSheet Spanish |SpecSheet Chinese | MucuSol Botanical SDS MucuSol is a liquid product developed for use in the drinking water of your poultry and swine populations as an aid in reducing respiratory congestion from mucus (phlegm).
RELATED ARTICLES:
Unblock Productivity Pressures During AMPV Challenges
Breathtaking Production PDF | ARTICLE
MucuSol & Uni-Sol Tag-Team for Respiratory Relief PDF
Efficacy of MucuSol in pigs with SIV and P multocida PDF | ARTICLE
Spanish PDF | ARTICLE
Mailing Address:
Animal Science Products, Inc
PO Drawer 631408
Nacogdoches, TX 75963 - 1408
Physical Address:
3418 Rayburn Drive
Nacogdoches, Texas 75961
Phone & Fax
800.657.2324
936.560.0003
936.560.0157
